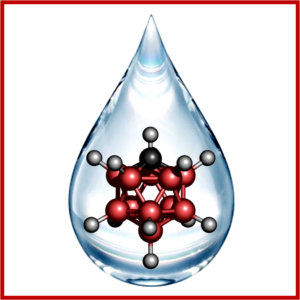
Stable ionic liquid electrolytes based on the carborane anion. Dr. Mega Kar, project leader at Monash notes access to reliable electricity is a big challenge in many parts of the world.
Monash University, a leading university in Australia, and the Toyota Research Institute North America based in Ann Arbor, Michigan claim that new battery materials discovery with non-volatile, non-flammable and electro-chemically stable electrolytes could be applied to batteries. The discovery is outlined in a study published in the international journal Energy and Environmental Science.
Technologies such as cellphones, laptops, and hybrid and electric cars rely on Li-ion batteries, of course. The configuration of such batteries is predominantly a graphite anode and a cobalt-based material cathode. The electrolyte typically consists of an organic electrolyte that is generally flammable and volatile as numerous vehicle and laptop fires can attest.
The Monash-TRINA group has found new electrolytes, based on liquid salts, that can be used in batteries to eliminate flammability and increase stability.
“We have also shown that these new electrolytes could provide enabling technologies of rechargeable magnesium and lithium metal batteries,” said study author Professor Doug MacFarlane from the Monash School of Chemistry. “These super stable electrolytes may also allow use of higher voltage materials in Li-ion batteries, increasing their energy storage capacity.”
TRINA principle scientist Rana Mohtadi, who is the project leader, said “the new materials were based on a small “icosahedral dice-shaped ion” called carborane anion, which is made of boron clusters. Research has shown recently that these materials are very stable and applicable beyond lithium battery chemistries. We wanted to exploit the stability of these anions to make ionic liquids that enable high-energy density batteries based on metals such as lithium and magnesium. In fact, this is the first to time show that ionic liquids can even function in magnesium batteries.”
Toyota Research Institute North America, a division of Toyota Motor North America Research & Development (TMNA R&D), aims to redefine next-generation cars as not simply a form of transportation, but – cliché alert – as a fully connected vehicle. In fact, since 2003, Toyota has been awarded more patents than any other automaker, including autonomous vehicle patents (more than 1,400). Globally, Toyota spends approximately $1 million per hour on R&D to ensure that Toyota “continuously develops cutting-edge, high-quality and appealing vehicles.”

About Ken Zino
Ken Zino, editor and publisher of AutoInformed, is a versatile auto industry participant with global experience spanning decades in print and broadcast journalism, as well as social media. He has automobile testing, marketing, public relations and communications experience. He is past president of The International Motor Press Assn, the Detroit Press Club, founding member and first President of the Automotive Press Assn. He is a member of APA, IMPA and the Midwest Automotive Press Assn.
He also brings an historical perspective while citing their contemporary relevance of the work of legendary auto writers such as Ken Purdy, Jim Dunne or Jerry Flint, or writers such as Red Smith, Mark Twain, Thomas Jefferson – all to bring perspective to a chaotic automotive universe.
Above all, decades after he first drove a car, Zino still revels in the sound of the exhaust as the throttle is blipped during a downshift and the driver’s rush that occurs when the entry, apex and exit points of a turn are smoothly and swiftly crossed. It’s the beginning of a perfect lap.
AutoInformed has an editorial philosophy that loves transportation machines of all kinds while promoting critical thinking about the future use of cars and trucks.
Zino builds AutoInformed from his background in automotive journalism starting at Hearst Publishing in New York City on Motor and MotorTech Magazines and car testing where he reviewed hundreds of vehicles in his decade-long stint as the Detroit Bureau Chief of Road & Track magazine. Zino has also worked in Europe, and Asia – now the largest automotive market in the world with China at its center.


Potential Performance Improvement in Energy-Dense Batteries Touted by Toyota
Stable ionic liquid electrolytes based on the carborane anion. Dr. Mega Kar, project leader at Monash notes access to reliable electricity is a big challenge in many parts of the world.
Monash University, a leading university in Australia, and the Toyota Research Institute North America based in Ann Arbor, Michigan claim that new battery materials discovery with non-volatile, non-flammable and electro-chemically stable electrolytes could be applied to batteries. The discovery is outlined in a study published in the international journal Energy and Environmental Science.
Technologies such as cellphones, laptops, and hybrid and electric cars rely on Li-ion batteries, of course. The configuration of such batteries is predominantly a graphite anode and a cobalt-based material cathode. The electrolyte typically consists of an organic electrolyte that is generally flammable and volatile as numerous vehicle and laptop fires can attest.
The Monash-TRINA group has found new electrolytes, based on liquid salts, that can be used in batteries to eliminate flammability and increase stability.
“We have also shown that these new electrolytes could provide enabling technologies of rechargeable magnesium and lithium metal batteries,” said study author Professor Doug MacFarlane from the Monash School of Chemistry. “These super stable electrolytes may also allow use of higher voltage materials in Li-ion batteries, increasing their energy storage capacity.”
TRINA principle scientist Rana Mohtadi, who is the project leader, said “the new materials were based on a small “icosahedral dice-shaped ion” called carborane anion, which is made of boron clusters. Research has shown recently that these materials are very stable and applicable beyond lithium battery chemistries. We wanted to exploit the stability of these anions to make ionic liquids that enable high-energy density batteries based on metals such as lithium and magnesium. In fact, this is the first to time show that ionic liquids can even function in magnesium batteries.”
Toyota Research Institute North America, a division of Toyota Motor North America Research & Development (TMNA R&D), aims to redefine next-generation cars as not simply a form of transportation, but – cliché alert – as a fully connected vehicle. In fact, since 2003, Toyota has been awarded more patents than any other automaker, including autonomous vehicle patents (more than 1,400). Globally, Toyota spends approximately $1 million per hour on R&D to ensure that Toyota “continuously develops cutting-edge, high-quality and appealing vehicles.”
About Ken Zino
Ken Zino, editor and publisher of AutoInformed, is a versatile auto industry participant with global experience spanning decades in print and broadcast journalism, as well as social media. He has automobile testing, marketing, public relations and communications experience. He is past president of The International Motor Press Assn, the Detroit Press Club, founding member and first President of the Automotive Press Assn. He is a member of APA, IMPA and the Midwest Automotive Press Assn. He also brings an historical perspective while citing their contemporary relevance of the work of legendary auto writers such as Ken Purdy, Jim Dunne or Jerry Flint, or writers such as Red Smith, Mark Twain, Thomas Jefferson – all to bring perspective to a chaotic automotive universe. Above all, decades after he first drove a car, Zino still revels in the sound of the exhaust as the throttle is blipped during a downshift and the driver’s rush that occurs when the entry, apex and exit points of a turn are smoothly and swiftly crossed. It’s the beginning of a perfect lap. AutoInformed has an editorial philosophy that loves transportation machines of all kinds while promoting critical thinking about the future use of cars and trucks. Zino builds AutoInformed from his background in automotive journalism starting at Hearst Publishing in New York City on Motor and MotorTech Magazines and car testing where he reviewed hundreds of vehicles in his decade-long stint as the Detroit Bureau Chief of Road & Track magazine. Zino has also worked in Europe, and Asia – now the largest automotive market in the world with China at its center.